C> is vulnerable to violent shock during transportation.
Modality Solutions is paving the way for the future of pharmaceutical transportation testing. The...
read Details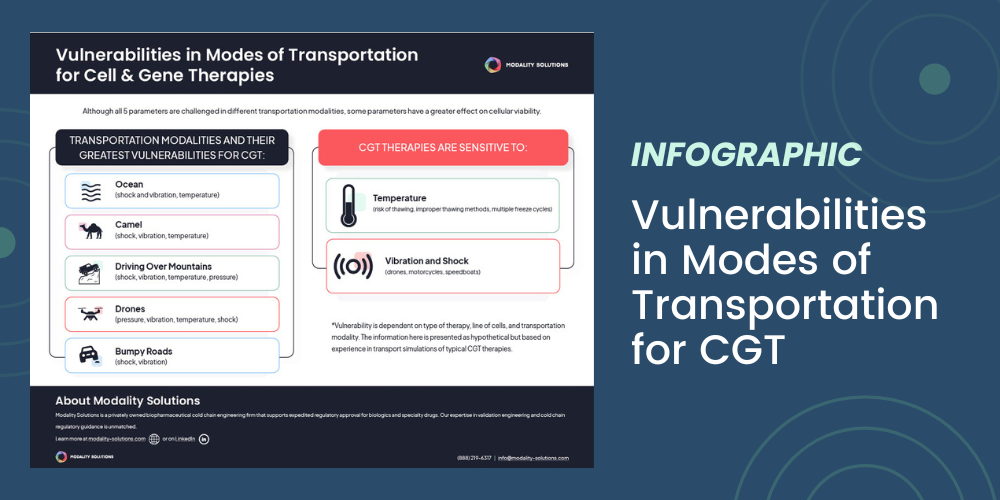

Monoclonal antibodies (mAbs) are clones of the antibodies that our body secretes naturally to combat germs and diseases. Our body will develop an antibody to specifically attack a certain antigen, which is the part of the diseased cell that the antibody will bind to. We commonly hear people say “you need to get sick to improve your immune system,” which is to say, when you get sick, your body develops antibodies to attack those diseases. Monoclonal antibodies, in particular, are clones of antibodies created artificially in labs and designed to specifically target certain antigens. (A lot of monoclonal antibody research is completed regarding cancer, developing specific antibodies to attack cancer cells.)
Fragments of the monoclonal antibodies are pieces of the antibody that pharmaceutical manufacturers biochemically or genetically separate from the parent antibody. What’s particularly interesting about mAb fragments is that they’re isolated to keep their function and specificity. They retain their ability to target a certain cell, even as a smaller and simpler unit.
MAb fragments essentially get rid of the excess that makes the unit too large to get to the cell. As a monoclonal antibody fragment, the unit can penetrate tissue better than a full mAb. It’s common knowledge that despite the fact mAbs originally come from the patient immune system, the body can trigger an immune response and attack what is meant to be a cure. mAb fragments are less likely than full mAbs to cause an immune response in the patient because of their size. (And they’re cheaper, faster, and easier to make.)
Because fragments are more specific, they can have a greater affinity for their target than the full-length monoclonal antibodies. They can also be linked to drugs for greater impact. For example, when battling cancer, the drug itself might not get to the tumor because it’s too large, the body flushes it out, or it deteriorates before it can get there. However, if you link the drug to the antibody fragment, the fragment will link to the tumor and carries the drug with it.
One of the biggest complications with monoclonal antibodies is aggregation, where mAbs will start to group together and form small particulates. Aggregation causes two core issues, 1) they lower the potency and efficacy of the therapeutic dose, and 2) trace amounts of aggregation can cause sever inflammation or even a fatal immune response. The bigger the mAb, there’s a higher possibility that the patient’s immune system will react to it.
With fragments, the aggregation becomes much worse because aggregation forms at the hydrophobic sections of the mAbs. These parts are attracted to one another and group together. Most of the therapeutic fragments that are formed are the hydrophobic sections of the full antibodies. In full antibodies, they’re most stable when they’re folded over on each other. Those hydrophobic sections can be hidden and encapsulated in a full mAb, but if they’re isolated, they’re more likely to aggregate together.
It’s not known how much of an effect the 5 transportation hazards (shock, vibration, pressure, humidity, and temperature) will have on aggregation of the fragments. There has been testing for full-length antibodies where researchers have found that shock experienced from dropping a package causes shock waves, which causes cavitation, which is the formation, growth, and collapse of cavities in the liquid solution of the antibodies.
After these air cavities collapse, they cause hot spots, which are areas of high temperature and pressure. These cause radicals to form in the mAb, which leads to structural changes, and then more aggregation.
Early research shows that shock and high temperatures cause additional aggregation, but it’s not been fully fleshed out, and there’s much more research to be done. Pharma manufacturers should be analyzing environmental hazards happening concurrently on mAb fragments to fully understand the impact.
At the same time, there’s minimal research available regarding the effects of aggregation on fragments. That’s dangerous for people who aren’t relying on transport simulation because if you’re using a real-world shipping test, you might not get the worst-case drop.
At Modality Solutions, we test in the 1% worst-case scenario for shock, vibration, pressure, and temperature. For instance, 99% of the time, your temperature is going to be within a certain range, but maybe 1% of the time the temperature (or pressure or shock) is more extreme, or maybe the real-world shipping tests aren’t analyzing the impact of all hazards concurrently. Modality Solutions takes care of that. We want to understand the full potential impact of hazards on aggregation for mAb fragments.
As we continue to test mAB fragments for pharmaceutical manufacturers, it’s likely that our engineers will conduct more thorough testing along more stringent standards. When we support companies by writing protocols, we’ll indicate the level of care that needs to be taken with a fragment, as opposed to a full-length mAB.
It is inevitable that the pharmaceutical industry will move towards the utilization of mAb fragments for specific therapies (especially in cancer research). The universal hope is that with a more concentrated therapy, the patient will experience fewer side effects due to a lower immune response from the fragment than the full mAb. These fragments also have the potential to be more effective for the patient, carrying the drug straight to the target area (like a tumor) instead of having to be processed through the body.
As the pharmaceutical industry pivots and innovates, so does Modality Solutions. Our team is constantly aware of upcoming trends and discoveries in the pharmaceutical spaces so that our Cold Chain Engineers and Cold Chain Consultants can have the most thorough, accurate testing in place possible, pleasing regulatory agencies and keeping patients safe. If you’re interested in talking with a Cold Chain expert, don’t hesitate to reach out to Modality Solutions.
Modality Solutions is paving the way for the future of pharmaceutical transportation testing. The...
read Details
In this guide, the experts from Modality Solutions discuss 13 keys you need to...
read Details
When your mAb or other biologic is moving through the Biologics License Application (BLA)...
read Details