AI vs. Humans: Can Artificial Intelligence Outsmart Cold Chain Experts?
10 Common Misconceptions of Cold Chain Validation, as Explained by AI, commentary by Gary...
read Details
Autologous cell therapy (ACT) is a therapeutic intervention that uses an individual’s cells, which are cultured and expanded outside the body, and reintroduced into the donor. Advantages of this approach include the minimization of risks from systemic immunological reactions, bio-incompatibility, and disease transmission associated with cells not cultivated from the patient. We intend to review the promising CAR T-Cell Therapy that uses apheresis collection to start the ACT process and address the potential risks your cold chain supply management faces.
Because of the inability to categorically define the nature and expression of biomolecules and factors that are present, the apheresis collection for CAR T-Cell therapy are inherently fragile and are highly sensitive to variations in manufacturing and cold supply chain procedures. Carefully controlling every aspect of how apheresis is handled is the best way to ensure that the full potential of this exciting innovation in immunotherapy is realized. Standardization of immunotherapy manufacturing, storage conditions, and cryopreservation are a stepwise approach and should allow the field to deliver efficient, competitive, and approved new therapeutic options for patients with still-unmet medical needs.
Supply chain risks include extrinsic cold chain factors, such as temperature, CO2 ingress, humidity, shock, and vibration occurring during transportation from collection site to manufacturer and storage conditions which may influence the metabolic activity of the cells and therefore the function, specificity, and efficacy of the patient’s treatment.
With the 2017 FDA approval of Kymriah (Merck) for the treatment of children with acute lymphoblastic leukemia (ALL), and Yescarta (Kite Pharma) for adults with advanced lymphomas, adoptive cell transfer (ACT), a specialized type of immunotherapy, has emerged as the front line in cancer treatment. [1]
With ACT, the patient’s own T cells are the major component of healing since they have the unique ability to initiate the immune system’s response to destroy cancer cells. A type of ACT making breakthroughs in targeted treatment is the chimeric antigen receptor T-Cell Therapy (CAR T-Cell). [2]
T cells are collected via autologous cell therapy, a complicated and multi-step process. The autologous cell therapy collection process has enabled breakthroughs in the treatment of cancer, but has also created challenges for regulatory officials, clinical workers, and supply chain professionals. [1] The collection takes place in a variety of clinical settings typically governed by local procedures. The time from T-cell harvest to infusion of CAR T-Cells varies from a few days to weeks [4]. CAR T-Cell Therapy is personalized medicine at its most elemental: a patient receives an exclusive treatment based upon their unique T cells.
To begin with, a patient typically undergoes blood cell collection or apheresis or a tumor cell collection. Blood cell collection is then assigned a unique identifier used throughout the manufacturing, distribution, and infusion process to ensure the patient receives the correct treatment. [3]
The apheresis sample is sent directly to the drug manufacturer, where the manufacturing process begins immediately. This collection could be shipped refrigerated and in some cases are cryogenically preserved at time of collection. In either case, strict temperature controls and monitoring are recommended to ensure high-quality starting material for downstream manufacturing processes. Qualified shipping containers, proper monitoring techniques, and validated procedural controls are key to ensuring bio-viability and patient safety. The process for receipt, storage, and distribution of the finished product is vital to success.
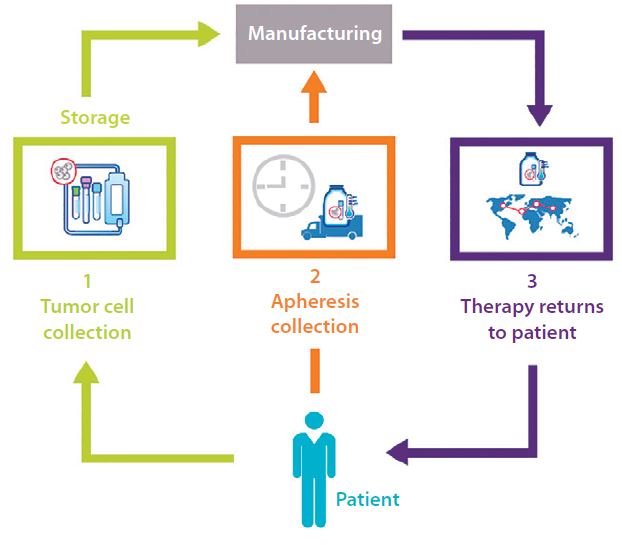
Figure 1: The typical process for a cancer-related autologous cell therapy [3]
Given the potential risks, personalized therapies require both a reliable and traceable chain of custody and chain of identity. Cancer patients undergoing immunotherapy have typically failed traditional methods of treatment, and their lives are literally in the hands of everyone involved – from the clinician collecting the sample to the material handler receiving the finished drug product into inventory.
Since the suitability of the patient’s harvest cells determine the reliability of cell assays the best practice is to cryopreserve the cells immediately after collection. Passive (non‐programmable), rate freezing has the advantages of lower cost, simplicity, and speed, and most importantly, facilitates the immediate cryopreservation of stem cell products avoiding potential pre‐freeze storage risks. [5]
The technology encompassing autologous cell therapy collection, storage, and processing has continued to evolve and merits a thorough review of current quality control expectations. FDA guidelines on who is qualified to pack the cells for initial shipment or the number of clinical investigators who should handle them upon return for patient infusion are evolving. Cryopreservation at time of collection needs adequate equipment and technical proficiency to monitor the frozen cells from collection site all the way to treatment application site to ensure reliable results and treatment efficacy.
Do you have an appropriate monitoring strategy? Have you optimized your monitoring and procedural controls and your qualification approach? Are you ready to provide the option of immediate cryopreservation to a patient whose cells are collected and ready for manufacturing their specialized immunotherapy treatment?
Your quality and service level agreements with partners are critical to your success.
By using simple measures such as standardization of documentation, including cold chain transportation validation with shock and vibration simulation validation master plans, sample handling protocols, and summary reports, you minimize the opportunity for error.
On-Demand Webinar: Cold Chain Validation Best Practices Including Immunotherapy Webinar
Blog: The cold chain challenges of today’s promising antibody-drug conjugates (ADCs)

References
1. Dana Farber Cancer Institute. Cellular Therapies Program. Dana Farber Cancer Institute. [Online] [Cited: May 11, 2019.] https://www.dana-farber.org/cellular-therapies-program/car-t-cell-therapy/faq-about-car-t-cell-therapy/.
2. US Department of Health and Human Services. NCI Dictionary of Cancer Terms. National Cancer Institute. [Online] [Cited: May 13, 2019.] https://www.cancer.gov/publications/dictionaries/cancer-terms/def/adoptive-cellular-therapy.
3. O’Donnell, Dan. The Cell Therapy Supply Chain: Logistical Considerations for Autologous Immunotherapies. BioProcess International. [Online] [Cited: March 25, 2019.] https://bioprocessintl.com/manufacturing/cell-therapies/the-cell-therapy-supply-chain-logistical-considerations-for-autologous-immunotherapies/.
4. Leukemia & Lymphoma Society. Facts About Chimeric Antigen Receptor (CAR) T-Cell Therapy. Leukemia and Lymphoma Society. [Online] [Cited: May 13, 2019.] Revised June 2018. https://www.lls.org/sites/default/files/National/USA/Pdf/Publications/FSHP1_CART_Factsheet_June2018_FINAL.pdf.
5. Watts, Michael, and Lynch, Davis J. British Journal of Haematology. Wiley Online Library. [Online] [Cited: May 21, 2019.] Immediate Cryopreservation is supported as a best practice at the beginning of autologous cell therapy smaple collection. https://onlinelibrary.wiley.com/doi/full/10.1111/bjh.14378.
6. Modality Solutions. The Challenging Supply Chain & Logistics of Immunotherapy Drugs. Modality-Solutions.com. [Online] [Cited: March 25, 2019.] https://www.modality-solutions.com/the-challenging-supply-chain-logistics-of-immunotherapy-drugs/.
10 Common Misconceptions of Cold Chain Validation, as Explained by AI, commentary by Gary...
read Details
When it comes to ensuring the integrity of temperature-sensitive products during transit, choosing the...
read Details
Getting your vaccine or therapeutic approved by the FDA is a significant accomplishment. But...
read Details