AI vs. Humans: Can Artificial Intelligence Outsmart Cold Chain Experts?
10 Common Misconceptions of Cold Chain Validation, as Explained by AI, commentary by Gary...
read Details

While blockchain is typically associated with bitcoin or other cryptocurrencies, it is actually a data format that is being implemented across industries. In the food supply industry, IBM has partnered with companies like Dole, Nestlé and Walmart in an effort to regulate food safety. IBM has also entered the jewelry market, as blockchaining enables consumers to track that their diamonds are being ethically sourced. Outside of industry, the United Nations and the World Identity Network announced an initiative known as “Blockchain for Humanity”, which will build a digital identity network for children without birth certificates.
In addition to these industries and organizations, life sciences companies are implementing this technology to increase security, prevent counterfeiting, and allow for better tracking of their products. A Health IT Analytics (2017) article reported that 22 percent of life science companies are presently using or experimenting with blockchain technology.1 A survey used in the same article indicates that more than 80 percent of those surveyed believe that blockchain usage will be widespread within five years. While it is difficult to fully understand the mathematics and networking behind blockchain, it is fairly easy to adopt and implement because the technology itself is open source. So, what is blockchain? And how can it possibly benefit the pharmaceutical serialization process?

Blockchain is a data format that uses previous entries in a sequence as part of the current entry. In a simplified sense, think of it like this:
If a serialization program gave a vial a serial number of “12345,” typically the next number would be “12346.” This is sequential serialization. With this process, a person could easily make counterfeits and give serial numbers that appear real. However, with blockchain serialization, the next number could potentially be, “45267” instead of “12346.” The first two numbers are the end of the first serial number, the third number is the “content” of the block, and the last two numbers are determined by an algorithm that is based on the rest of the numbers in the block. The number after that would be “67390.” Both of these steps used the same, incredibly simple, one-step algorithm to generate, and yet it is difficult to guess what the next number in the sequence would be. Because blockchain requires a person to not only know every serial number in sequence, but also the algorithm to determine the next set of numbers, it is near impossible to guess or create a valid serial number.
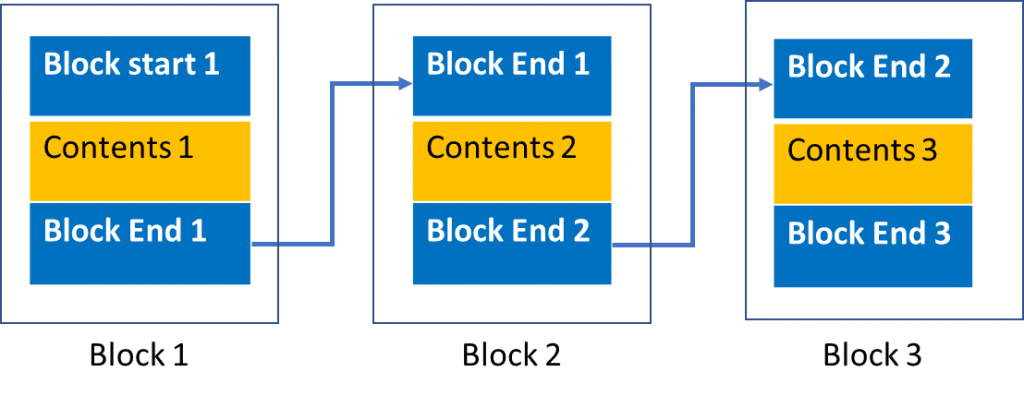
How does this benefit the pharmaceutical serialization process? Counterfeit pharmaceuticals rely on being able to guess serial numbers to create a product that seems legitimate. In 2012, it was revealed that counterfeit versions of Roche’s cancer drug Avastin had been circulating through the United States. Development of copying and printing technologies along with the ability of online pharmacies to conceal the source of their products enable counterfeiting.2 If someone has a single vial from a sequential serialization program, they can make a batch of serial numbers that are valid. Because the serial number is one of the only ways to verify a product, these counterfeits will make it to the patient. Blockchain prevents this by making it effectively impossible to guess a valid number.
There is actually another way blockchain technology can help with serialization. The other part of serialization that is important is creating traceability on where the product has been. Track and trace technologies, like serialization, have allowed pharmaceuticals to be followed down the supply chain. A few examples of blockchain being used for traceability exist transnationally. In April of 2017, IBM launched a partnership with Chinese supply chain management firm Easysight Supply Chain Management to introduce the Yijian Blockchain Technology Application System.3 Imperial Logistics has also made a notable stride, as they have partnered with One Network Enterprises to provide a serialization and authentication process for distribution.4 Drug pedigrees are another form of track and trace technology that record the details of drug distribution until it reaches the dispenser. The final regulations for pedigrees were drafted in the United States in 1999.5 More legislative action was taken toward pharmaceutical traceability in 2013, when US Congress enacted the Drug Supply Chain Security Act (DCSA). The Act outlines steps to build an electronic system to identify and trace drugs that are distributed throughout the United States.6 This system would also aim to improve detection and removal of counterfeit drugs from US supply chains.

The serialization system, although newer than pedigrees, is promising in protecting pharmaceuticals. Applying blockchain technology can help with the traceability function of serialization. Besides the serial numbers themselves, the data on what vials are going where can also be in blockchain format. Again, you begin with a special number at the beginning. Then, the block “contents” would say something like this:
“Vial 1 goes from Point A to Point C – confirmed by point C”
“Vial 3 goes from Point A to Point B – confirmed by point B”
“Vial 1 goes from Point C to Point B – confirmed by point B”
“Vial 2 Goes from Point C to Point A – confirmed by point A”
When looking at this list of contents, you can see every movement every vial makes and you can see who confirmed those movements. After so many lines of content, the ending number will be determined for the block. Again, this number will be a function of the beginning special number, but also the content itself. Once that number is determined, the next block of content begins.
Now think of how this works in practice. Say someone tried to change the data to say that vial 1 ended at point D instead. When that is changed and the block is recompiled, the ending number will change. This change will change every block afterwards, which will signal to everyone looking at the records that the data in the block is counterfeit.
Each transaction is signed by the people who made them. Then, the next part of blockchain data comes in to play. When a block is “closed” by the ending number and added to the chain, that block is then broadcasted to everyone on the network. If that block is not compatible with the previous block someone received, they will know there is a counterfeit record in the chain and can take steps to remediate it. The other way this is beneficial is that if someone receives extra vials, they can check the data in the block chain to see where those vials came from, where they should be, or if they are counterfeit product.
Until recently, encrypting serialization this way is not something that was even considered. However, with the rise of imitation and counterfeit products, as well as potential theft, patient safety is becoming a significant issue along with the potential economic harm that can come from bad product.
Sources:
1. https://healthitanalytics.com/news/83-of-life-science-execs-expect-healthcare-blockchain-in-5-years
2. https://www.reuters.com/article/us-drugs-counterfeits/fake-avastin-shows-very-little-protects-drug-supply-idUSBRE82B00120120312
3.https://www.pharmaceutical-technology.com/features/blockchain-pharma-opportunities-supply-chain/
4.https://www.onenetwork.com/2018/03/blockchain-serialization-authentication-medical-supply-chain/
5.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3617666/
6.https://www.fda.gov/Drugs/DrugSafety/DrugIntegrityandSupplyChainSecurity/DrugSupplyChainSecurityAct/
10 Common Misconceptions of Cold Chain Validation, as Explained by AI, commentary by Gary...
read Details
When it comes to ensuring the integrity of temperature-sensitive products during transit, choosing the...
read Details
Getting your vaccine or therapeutic approved by the FDA is a significant accomplishment. But...
read Details