How to Select the Right Thermal Packaging for Your Cold Chain
When it comes to ensuring the integrity of temperature-sensitive products during transit, choosing the...
read Details

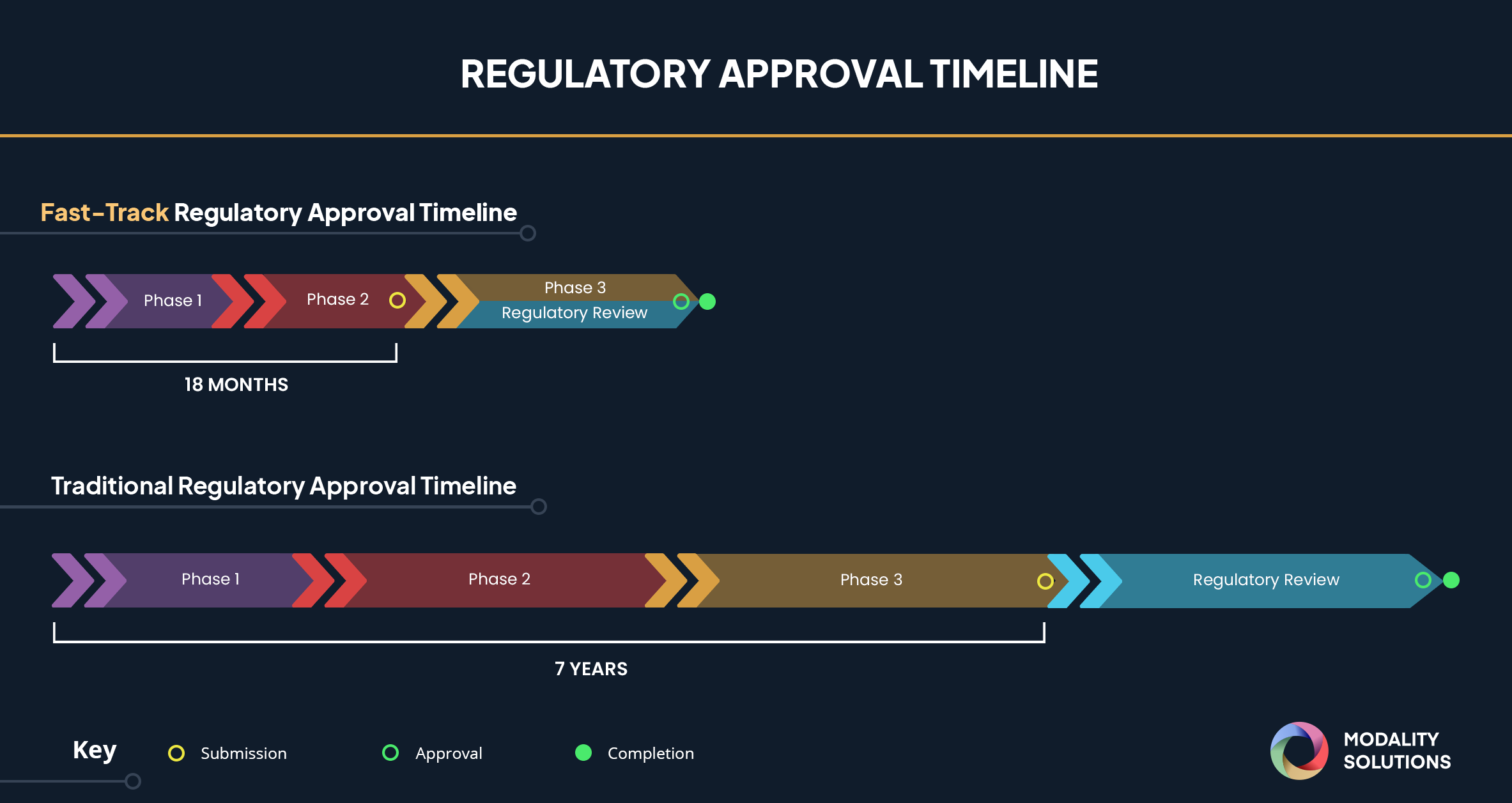
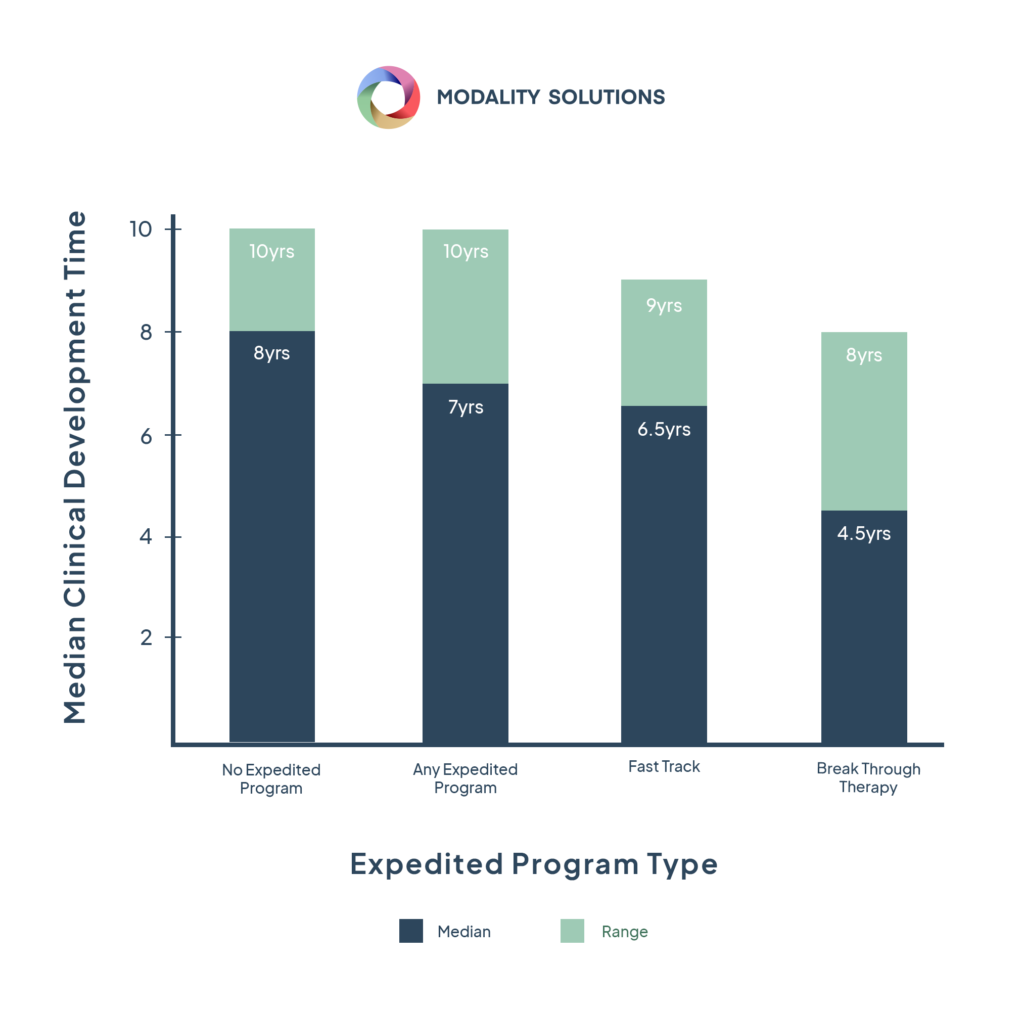
FDA Fast-Track designation compresses approximately eight years of development and validation activities into roughly 18 months. To meet this accelerated timeline, we recommend performing transportation simulation testing for cold chain validation as early as possible. Simulation testing provides robust, repeatable, and reliable results in four days or less for most proposed shipping lanes. Real-world shipments cannot be assured proper control of testing parameters and cannot be used afterward for root cause analysis if drug product formulation stability issues are uncovered.
More than 30 countries have adopted or amended Good Distribution Practices for pharmaceuticals in the past decade.i While the specifics vary, they all advise some degree of environmental or condition monitoring and tracking to ensure therapeutics and diagnostics are handled in a way that ensures their safety and efficacy.
U.S. Pharmacopeia Good Storage and Distribution Practices for Drug Products Chapter 1079 advises operational and performance testing for products, as well as temperature profiling studies for transportation and warehouses. iiASTM D4169, “Standard Practices for Performance Testing of Shipping Containers and Systems” addresses packaging durability for each shipping mode.iii
The EU’s Guidelines on Good Distribution Practice of medicinal products for human use (2013/C 343/01) are based on Article 84 and Article 85b(3) of Directive 2001/83/EC. Chapter 9 of that document calls for a risk-based approach for transportation that demonstrates that “medicines have not been exposed to conditions that may compromise their quality and integrity.”iv
Health Canada recommends using temperature monitoring devices where appropriate. It notes, “Temperature excursions outside of their respective labeled storage conditions for brief periods may be acceptable provided stability data and scientific/technical justification exist demonstrating that product quality is not affected.”v
To follow any of these guidelines, pharmaceutical manufacturers need transportation testing that simulates the temperature, humidity, pressure, vibration, and shock a biopharmaceutical product may experience during transit and storage. Unless those conditions are tested concurrently, the simulation won’t truly reflect real-world hazards. And if these hazards are not tested in a controlled laboratory testing, there is no guarantee that the “edges” of the operating space have been tested.
Fast-Track designation is granted to approximately 60% of drug formulation approved last year, so early transportation simulation testing is vital. Compressing the usual seven to eight-year timeline into 18 months requires biopharmaceutical companies to work even more efficiently. For product and package testing, that means simulating worst-case shipping conditions to meet this accelerated timeline while obtaining reliable results.
The drug product’s stability specifications are the starting point. Modality Solutions tests at the edge of the operating space using worst-case conditions for all five major environmental hazards concurrently to determine how long the product can be exposed to combinations of temperature, humidity, pressure, vibration, and shock before the drug product formulation’s physical or chemical integrity is affected. This “edge of the operating space” testing strategy cannot be executed with ‘real-world’ shipping studies, which ensures you cannot thoroughly test the test your drug product stability in shipping studies alone.
We also perform a functionality test on the selected thermal packaging to simulate the maximum time the product could be in transit, and formulation integrity is not compromised from the point of manufacture to the distributor, pharmacy, or end-user. Based on test results, the thermal packaging solutions selected could be confirmed or optimized.
Notably, Modality Solutions tests products and packaging against multiple hazards simultaneously to ensure the actual risks and worst-case exposures during transportation are investigated. Results obtained are more realistic, than is possible when testing each parameter separately and more reliable than real-world shipping studies.
Modality Solutions has performed simulation testing for more than 70 pharmaceutical products, presentations, and indications and provided the data to regulators to date. Each of those cold chain process validation submissions has been reviewed and approved by regulators around the world.
Performing thorough transportation simulation testing as early as possible ensures that if there are any surprises – such as formulations that are less stable than anticipated or packaging that is less robust than promised – pharmaceutical developers have time to address them. Consequently, regulatory submission packages can be complete, accurate, and well-documented.
At Modality Solutions, our team of experts is attuned to regulatory guidance around Good Distribution Practices, and we have the testing capabilities to simulate and evaluate the effects of environmental hazards throughout the supply chain. We offer robust transportation simulation testing as well as clinical trial cold chain monitoring, site compliance, safety review, end-user training, and project management. Our goal is to establish a collaborative relationship with our clients to deliver fast, successful, and scientifically sound supply chain cold chain solutions for clinical trials globally.
When you’re ready to test your products against even the harshest shipping conditions, contact us. Modality Solutions is here to help.
i A Global Review of Good Distribution Practices, IQPC, https://www.iqpc.com/media/8378/34072.pdf
ii U.S. Pharmacopeia Good Storage and Distribution Practices for Drug Products, chapter 1079, http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1079.html
iii Standard Practice for Performance Testing of Shipping Containers and Systems. ASTM, https://www.document-center.com/standards/show/ASTM-D4169
iv Guidelines of 5 November 2013 on Good Distribution Practice of medicinal products for human use(2013/C 343/01), European Commission https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2013:343:0001:0014:EN:PDF
v Guidelines for Temperature Control of Drug Products during Storage andTransportation (GUI-0069), HealthCanada,https://www.usp.org/sites/default/files/usp/document/workshops/guidelines_for_temperature_control_of_drug_products_during_storage_and_transportation_gui-0069_health_canada_2011.pdf
When it comes to ensuring the integrity of temperature-sensitive products during transit, choosing the...
read Details
Getting your vaccine or therapeutic approved by the FDA is a significant accomplishment. But...
read Details
This six-part article series explores Cold Chain Process Validation, addressing the multifaceted challenges and...
read Details