Part 2: Expanding Shipping Temperatures Without Sacrificing Quality: A Guide to Transport Validation and PQ
Demonstrating Product Quality All pharmaceutical manufacturers must ensure that the safety and efficacy of...
read Details

On July 31st, 2020 the FDA has approved tafasitamab-cxix, officially named Monjuvi®, in combination with Lenalidomide as a second-line treatment for adult patients with relapsed or Refractory Diffuse Large B-cell Lymphoma (DLBCL) for our client MorphoSys. We are very proud of their fantastic accomplishment and excited about our small role in their success.
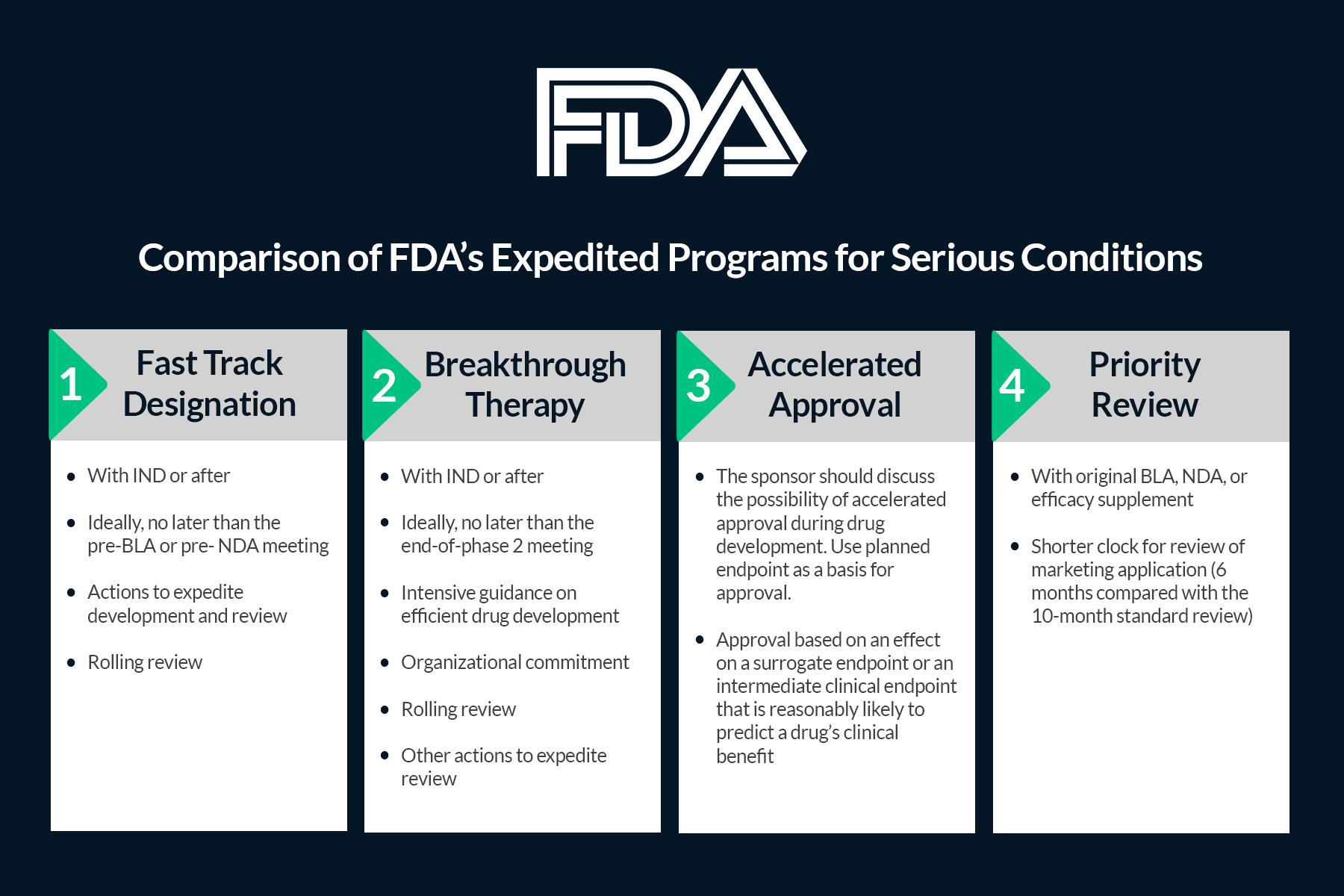
Monjuvi, in combination with Lenalidomide, addresses a high unmet medical need and qualified for the FDA’s Fast Track, Priority Review, and Breakthrough Therapy designations. These designations make the filing process, including cold chain validation, more challenging than other filings because of the compressed timelines and competing priorities for both material and intellectual capital across the organization.
Modality Solutions incorporated existing stability and engineering studies into a comprehensive cold chain validation strategy that was shared with regulators early in the approval process. Once the strategy was agreed upon, our focus shifted to creating data on drug product quality during transport.
Transport simulation testing provides the fastest and most effective approach to cold chain validation of drug product available. After five days of continuous stressing of drug candidate Monjuvi at the edges of safe operating space, MorphoSys could begin analytical testing of the stressed drug product immediately afterward.
This proven approach was shared up-front with their regulatory partners, and the drug product quality data was accepted without comment. This focused and accelerated pathway to approval has again been shown to be an industry best practice for biologics in general and monoclonal antibodies in particular.
DLBCL is the most common type of non-Hodgkin lymphoma in adults worldwidei, characterized by rapidly growing masses of malignant B-cells in the lymph nodes, spleen, liver, bone marrow, or other organs. It is an aggressive disease with about one in three patients not responding to initial therapy or relapsing thereafter.ii In the United States each year approximately 10,000 patients are diagnosed with relapsed or refractory DLBCL who are not eligible for ASCT.iii
FDA Breakthrough Therapy designation is intended to expedite development and review of drug candidates. It is granted if preliminary clinical evidence indicates that the drug candidate may demonstrate substantial improvement over existing therapies in the treatment of a serious or life-threatening disease. The Biologics License Application (BLA) for Monjuvi was granted Priority Review and approved under the FDA’s Accelerated Approval program.
In January 2020, MorphoSys and Incyte entered into a collaboration and licensing agreement to further develop and commercialize Monjuvi globally. Monjuvi will be co-commercialized by Incyte and MorphoSys in the United States. Incyte has exclusive commercialization rights outside the United States.
MorphoSys (NASDAQ:MOR) is a commercial-stage biopharmaceutical company dedicated to the discovery, development, and commercialization of exceptional, innovative therapies for patients suffering from cancer. Headquartered near Munich, Germany, the MorphoSys group includes a fully owned U.S. subsidiary MorphoSys U.S. Inc.iv
References
i. Sarkozy C, et al. Management of relapsed/refractory DLBCL. Best Practice Research & Clinical Haematology. 2018 31:209-16. doi.org/10.1016/j.beha.2018.07.014.
ii. Skrabek P, et al. Emerging therapies for the treatment of relapsed or refractory diffuse large B cell lymphoma. Current Oncology. 2019 26(4): 253-265. doi.org/10.3747/co.26.5421.
iii. DRG Epidemiology data.
iv. www.morphosys.com
Demonstrating Product Quality All pharmaceutical manufacturers must ensure that the safety and efficacy of...
read Details
Introduction The integrity of supply chain management is pivotal in the demanding realm of...
read Details
Entering the U.S. market comes with a unique set of regulatory challenges, particularly when...
read Details