Vertex announces UK approval for CASGEVY™
Congratulations to Vertex for their UK approval for CASGEVY™, a CRISPR/Cas9 gene-edited therapy, for...
read Details

Gary Hutchinson
Daniel J. Littlefield
The race for safe, efficacious COVID-19 vaccines is on, with an estimated 66 vaccine candidates in the clinical trial phase and many more in the preclinical drug development phase worldwide.1 While there is great urgency to find effective preventative measures and therapeutics for SARS-CoV-2, the virus that causes COVID-19, the drug discovery and manufacturing development process is just one piece of the puzzle. Once a vaccine candidate has been deemed efficacious and safe, has received regulatory approval, and has been manufactured in the significant quantities needed, another hurdle remains: Transporting the vaccine in a manner that preserves its integrity.
The cold chain requirements for COVID-19 vaccines will present a myriad of challenges. Fortunately, the industry’s experience with other modern vaccines, combined with technology-based transport validation solutions, provides a roadmap for successfully transporting these vital drugs.
The complexities of transporting a COVID-19 vaccine begin from the nature of these therapies and the challenges associated with any therapy developed on an accelerated timeline.
Traditional vaccines – like those designed to eradicate childhood diseases – use either an inactivated virus (as with polio, influenza, hepatitis A, and rabies) or an attenuated virus (as with measles, mumps, and chickenpox) to spur antibody development. Modern vaccines take a more sophisticated approach, using large, complex, fragile molecules to power the immune system to target and thwart a virus or other disease. The larger the molecule, the more susceptible it is to damage, notwithstanding the limited protection provided by the molecule’s lipid proteins.
 As with other large molecules, a vaccine is particularly sensitive to shock and vibration – two hazards that can jeopardize drug safety and efficacy. Vibration (which involves repetitive, low-amplitude impact) can serve as an energy input into a large, three-dimensional molecule, with the potential to alter it chemically. Shock (which involves short-duration, high-amplitude impact) can shear off part of the molecule, reducing its efficacy or introducing particulates that can cause adverse events.
As with other large molecules, a vaccine is particularly sensitive to shock and vibration – two hazards that can jeopardize drug safety and efficacy. Vibration (which involves repetitive, low-amplitude impact) can serve as an energy input into a large, three-dimensional molecule, with the potential to alter it chemically. Shock (which involves short-duration, high-amplitude impact) can shear off part of the molecule, reducing its efficacy or introducing particulates that can cause adverse events.
In addition to the complexities of any large molecule drug, COVID-19 vaccines will pose other logistical issues. Given the urgent need to stem the pandemic’s tide, these vaccines are being developed under the Coronavirus Treatment Acceleration Program (CTAP) specially created by the US Food & Drug Administration (FDA) to move new treatments to patients as quickly as possible. When vaccines and therapeutics move through the drug development and regulatory processes on an accelerated timeline, researchers tend to take a conservative approach, particularly when it comes to temperature tolerance.
Any biologic is sensitive to temperature, so tight temperature controls are always necessary to maintain their integrity. Because the first-round COVID-19 vaccine candidates were developed on a fast track, employing conservative formulation stability strategies, they will likely need to be stored in a deep-frozen state, typically defined as below -60°C. From a transportation standpoint, that usually means packing and shipping the vaccine in dry ice. By comparison, many established vaccines can be safely stored and transported at refrigerated temperatures of 2-8° C.
Another important consideration in transportation is a good understanding of the interaction of temperature, vibration, and shock. While any of these individually can cause product damage, they do not necessarily work independently of each other. This possible interaction emphasizes the importance of concurrent testing to investigate those transport activities which can result in any combination of significant vibration activity (e.g., long transportation lanes), temperature excursions, and multiple shock events (e.g., frequent manual handling or mode changes).
Controlling temperature and reducing the risk of vibration and shock throughout the cold chain is of the utmost importance when transporting modern vaccines and other therapeutics. While the safe transportation of COVID-19 vaccines will prove difficult, the industry’s experience with the Ebola outbreak in sub-Saharan Africa in 2015 demonstrates that it can be done effectively.
Much like COVID-19, the Ebola vaccine required storage in a deep-frozen state to avoid damage and preserve integrity. Once properly thawed and reconstituted, it could only be refrigerated for up to 12 hours prior to dosing. The associated transportation and distribution problems were successfully solved, demonstrating that supply chain networks can be designed, executed, and monitored amidst very formidable logistical obstacles. Though the COVID-19 pandemic is occurring on a much larger scale than the Ebola outbreak, the blueprint for its safe transport exists.
Safe and rapid distribution of a vaccine is critical to stemming the tide of the coronavirus pandemic. But before an approved COVID-19 vaccine can be shipped to healthcare providers, regulators must be assured that the pharmaceutical company’s cold chain plan is sound and feasible. Among their requirements, regulators such as the FDA require pharmaceutical companies and other drug makers to perform transport validation studies on the final formulation of the drug, which typically happens concurrent with phase III clinical trials. Additionally, initial transport validation may be done on engineering batches or before a final formulation decision is made, enabling the company to appraise multiple candidate formulations early in the drug development process.
This is the point at which the scientists’ drug discovery and development work is handed off to engineers and logisticians to determine how to protect the drug throughout the cold chain. Through rigorous risk assessments, these professionals work to find the optimum intersection of the drug product and the supply chain, demonstrating to regulators that the drug can be transported to the point of use on a large scale, while maintaining its safety and efficacy.
While the regulations vary by country, any developer of a COVID-19 vaccine or therapy will be required to demonstrate that it will maintain a chain of custody throughout the supply chain by employing consistent, repeatable processes that preserve the vaccine’s integrity. Transport validation is the critical part of that requirement.
Historically, transport validation for vaccines and other therapies was done in real-world settings – placing packages filled with a drug product on trucks and planes and sending them across the globe. Over time, the industry discovered that real-world shipments testing has inherent limitations.
Consider this example: A COVID-19 vaccine is driven by truck to an airport in Los Angeles, California, then flown from Los Angeles to Sydney, Australia, then driven to a clinic 15 miles away. That vaccine has endured a long flight, but it probably has not been subject to significant shock events since it was only dropped off and picked up a couple of times. If the same vaccine is driven by truck from Temecula,
California to San Diego, then flown to Louisville, Kentucky, where it takes a connecting flight to Columbus, Ohio and is then driven by truck to Marietta, Ohio, it has a greater chance of enduring shock because it has been picked up and dropped off more often. These two scenarios present very different risks, underscoring the fact that it is unlikely for real-world testing to simulate the many scenarios a drug might encounter in transit.
Critical feedback from the FDA and other regulators has created an incentive for pharmaceutical and biotech companies to overcome these limitations by conducting transport validation using more rigorous methodologies. Over time, transport validation testing has evolved and advanced significantly with the help of technology. Today, the makers of COVID-19 vaccines and other biologics can use technology-based simulation approaches to test the drug’s physical and chemical integrity, based on the actual hazards that will occur in the field, to ensure there will be no risk of degradation during transit.
Degradation of a COVID-19 vaccine can result from two primary causes: A change in chemical stability (e.g., the chemical properties of the molecule are altered) or a change in physical stability (e.g., a portion of the molecule breaks off and turns into particulates, or the vaccine’s lipid protein “unravels” too much while deep-frozen and does not return to its original state once thawed for dosing).
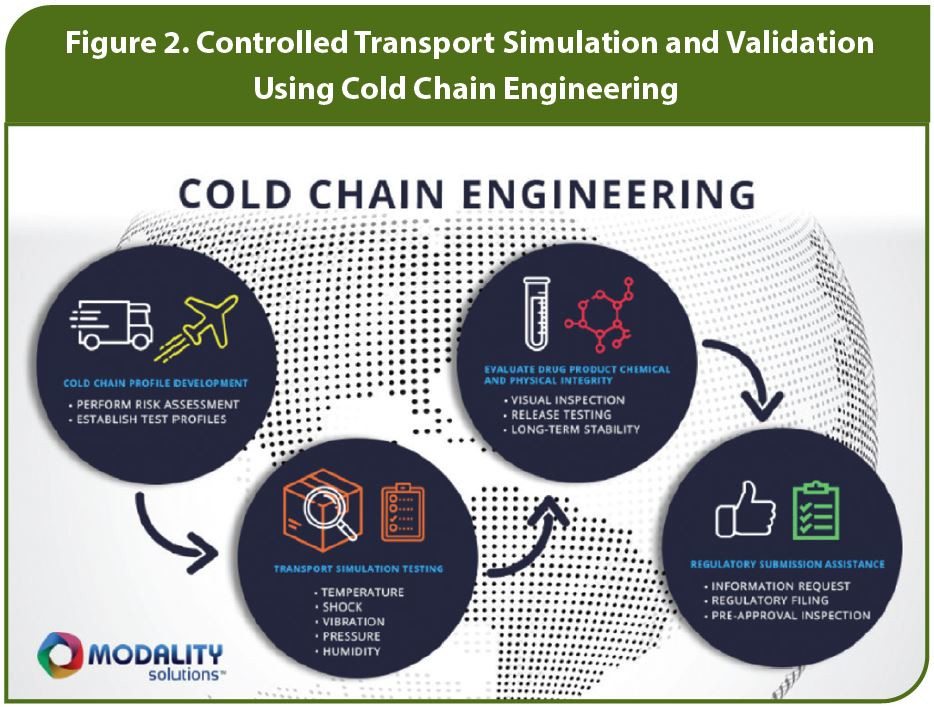
A transport simulation study can determine if degradation occurs after concurrent exposure to the five potential hazards drugs may encounter in transit: temperature, shock, vibration, pressure, and humidity. Typically, the study is preceded by a risk assessment to determine which risks are the highest priority for this vaccine or other drug, which then informs the test design and implementation. Deep knowledge of the shipping lanes the vaccine will eventually travel through is also essential to ensuring the test reflects real-world conditions.
With a valid transport test designed, logisticians and engineers can use simulation technology to test all five hazards on a worst-case scenario basis, concurrently, in a controlled lab setting, under repeatable conditions. For instance, various temperature fluctuations can be tested, varying levels and duration of vibration can be simulated, and shock events (such as package drops) can be done at different frequencies. This controlled testing environment yields data that can help assure pharmaceutical companies and regulators that no matter what happens in transit in the real world, the integrity, efficacy, and safety profile of the COVID-19 vaccine will be maintained.
Transport simulation studies also prove useful in situations where the drug is in short supply (as is the case with the early COVID-19 vaccine candidates) or is expensive. Given the scale of the pandemic and the urgency of stemming its spread, every ounce of COVID-19 vaccine is essential to preserve; there is no room for waste. Conducting a controlled transport validation in which multiple hazards are tested at their peak, concurrently, reduces the total amount of vaccine material needed. Additionally, because COVID-19 vaccine development is in its early stages there are still knowledge gaps, making it even more important to obtain as much information about the material as efficiently as possible.
Beyond meeting the regulatory requirement to demonstrate product robustness under worst-case hazard conditions, the data obtained from transport validation helps guide pharmaceutical companies on how to move forward with their cold chain network. Ideally, the testing will find that the vaccine maintained its chemical and physical integrity when subjected to the rigors of worst-case concurrent hazards.
In the event that transport validation uncovers potential risks in the operating plan, the company now has the information to develop risk mitigation measures. Mitigation is especially critical under the Coronavirus Treatment Acceleration Program (CTAP). There simply may not be time or resources to change the formulation, so other measures may be more feasible, such as changes to the packaging design or materials.
Once the transportation plan is validated and accepted by regulators, it is important to establish a program of monitoring and controls to ensure the vaccine reaches the front lines with its safety and efficacy profile intact. In the early stages of COVID-19 vaccine distribution, monitoring and controls will prove essential until additional stability data is available to support a wider acceptable temperature range. While these early vaccines, and other mRNA-based vaccines, will require deep-frozen temperatures for initial storage and transportation, they will also require dilution and thawing before dosing. After preparation for dosing, the temperature requirements switch to refrigerated temperatures (2 to 8° C), but the vaccine can only be kept at this temperature for a maximum of 24 to 48 hours before dosing. These complex temperature requirements require significant monitoring and controls to confirm the proper temperature and storage duration.
While the earliest COVID-19 vaccine candidates will require the most restrictive temperature controls, the next generation vaccines will benefit from a larger body of knowledge about their stability boundaries, opening the door to some formulations that will be stable at refrigerated temperatures, which the supply chain has a greater capacity to handle. For example, viral vector-based vaccines have less restrictive temperature and duration requirements, making them stable for months and or even years at refrigerated temperatures. This temperature range is more suitable to long-term vaccination and therapy programs, which COVID-19 vaccines will inevitably progress to over time. The data provided by transport simulation studies and real-world shipping tests will be integral to providing vaccine developers with the insights to develop such formulations.
However, challenges still exist when transporting vaccines at refrigerated temperatures. It will be critical to test that the refrigerated drug will withstand the hazards of shipment and that the formulation remains unchanged, through consistent and repeatable transport validation. When testing is done under the controlled conditions of a lab environment, it is feasible to repeat the same conditions tested with an early, deep-frozen formulation and compare the results to the new refrigerator-stable drug. Such a head-to-head comparison provides greater assurance to regulators that the proper controls are in place for safe transport. In turn, the challenges of safely transporting and distributing COVID-19 vaccines will become more than surmountable, helping to speed the delivery of these vital preventive medicines to people globally.
1. Corum. J., Wee, S., & Zimmer, C. (2020, October 28), Coronavirus Vaccine Tracker, The New York Times.
Gary Hutchinson, President of Modality Solutions, is an expert in controlled-environment logistics. He has deep expertise in understanding the environment and infrastructure, designing controls, and creating monitoring systems. Gary’s background in the life sciences field includes a focus on biologics, tissue heart valves, cardiac monitoring equipment, and perfusion systems. www.modality-solutions.com
Daniel Littlefield, Principal of Modality Solutions, is an expert in process improvement, risk assessment, safety, and security. Currently head of the organization’s engineering management activities, he has successfully led clinical trial operations and assessments worldwide. Daniel’s career includes a variety of technical and supervisory roles in research and operations. www.modality-solutions.com
Congratulations to Vertex for their UK approval for CASGEVY™, a CRISPR/Cas9 gene-edited therapy, for...
read Details
Congratulations to Melinta Therapeutics for their FDA Approval of REZZAYO™ for the Treatment of...
read Details
Congratulations to Iveric Bio for their FDA approval for IZERVAY™️️, a new treatment for...
read Details