Unlocking FDA Insights: Exploring Open Data Files for Shipping Validation
Are you aware of the open data files published online by the FDA? This...
read Details

It’s estimated that seven out of ten pharmaceutical products in the US require temperature control during transportation1, a trend that’s escalated with the rapid growth in temperature-sensitive therapeutics like large molecule, protein-based biologics. With many biopharmaceutical products now requiring storage at consistent temperatures within the range of 2° to 8° Celsius—and some requiring a deep-frozen state of -60° Celsius or below—the integrity of the biopharmaceutical cold chain is more vital than ever.
When temperature excursions occur during the shipment and storage of controlled-temperature drug products or during clinical trials, the product’s stability, efficacy, and safety can be at risk. That makes it essential for pharmaceutical companies to make the most informed decisions about how to resolve temperature excursions and handle drug products exposed to such excursions. Our experience helping a leading biopharmaceutical company address this challenge demonstrates the value of a data-driven, analytical approach to identifying and mitigating cold chain temperature excursions.
Reducing the risks associated with temperature excursions during shipment and storage is essential to protecting controlled temperature drug products. But across a global, complex biopharmaceutical supply chain, the tasks of monitoring and reporting on temperature excursions, managing temperature excursion data, and mitigating temperature-related issues promptly are becoming much more challenging.
Speed is of the essence when it comes to detecting that a temperature excursion has occurred, identifying the root cause, and determining the best course of action to ensure a safe, efficacious product makes it to the patient on time. That’s especially true for personalized medicines, such as cell and gene therapies that use the patient’s biological material as the therapeutic. When the treatment is costly to produce and in scarce supply, quickly identifying and correcting temperature excursions is a must.
Minimizing the impact of temperature excursions a drug product is exposed to in transit—and taking fast action in the event they occur—requires an effective and efficient temperature excursion management process that includes these critical steps:
Unfortunately, it’s common for various obstacles to jeopardize this process, including language barriers and other communication challenges among staff and time zone differences that cause delays in sharing and responding to temperature excursion notifications. The seemingly simple act of providing the necessary temperature excursion data to the staff member with the proper skill set to analyze it is often one of the most significant impediments. Using the results of that analysis to make an informed decision on whether to keep, quarantine, or discard and replace the affected shipment requires that the right person with the ability and authority to make that decision is notified quickly and equipped with actionable information.
A major biopharmaceutical company was experiencing these very problems when addressing temperature excursions for its controlled temperature drugs. After struggling with the best way to manage temperature excursion data, the company asked Modality Solutions to assess the situation and recommend a solution based on our in-depth experience evaluating and optimizing the biopharmaceutical cold chain.
Our assessment revelated that the company’s existing process for managing and mitigating temperature excursions lacked the decision-making data or tools needed for a fast, effective response. Erring on the side of caution, in some cases the company discarded shipments affected by temperature excursions and replaced them with emergency shipments in the absence of actionable data. By assuming the worst-case scenario, the company lost time and money and wasted resources whenever a temperature excursion occurred. The staff investigated each excursion after the fact but in a reactive mode.
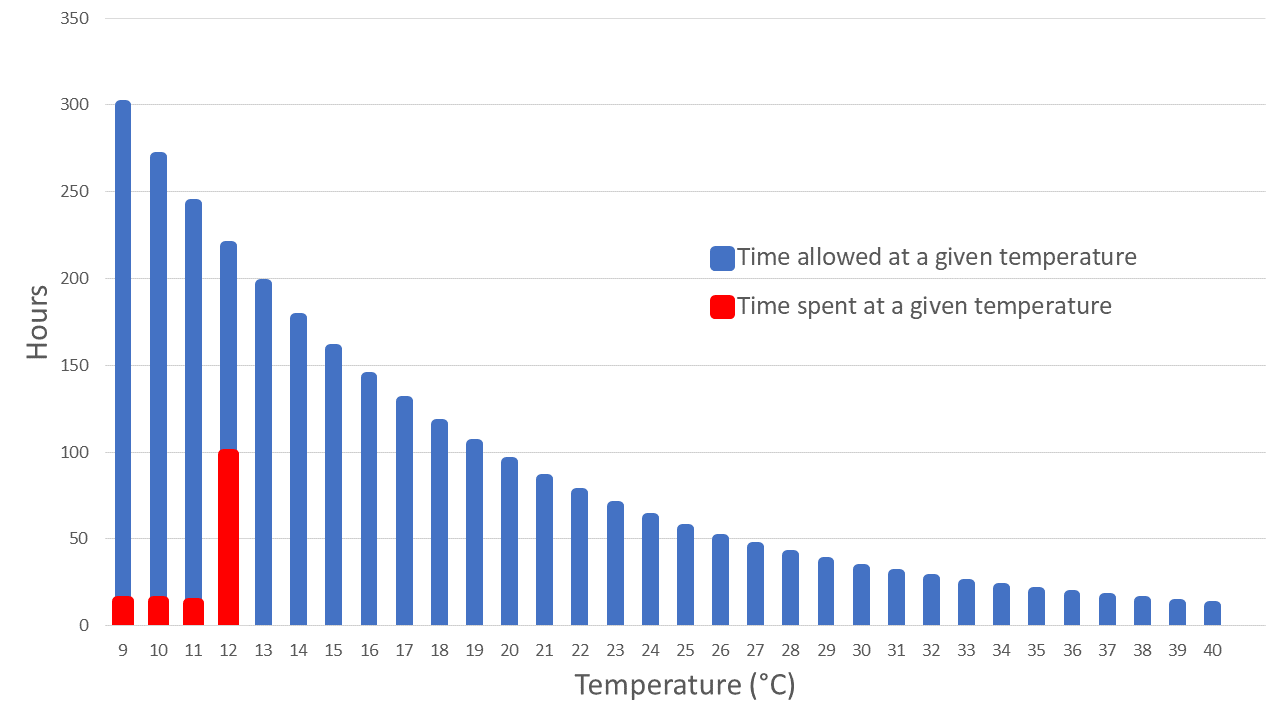
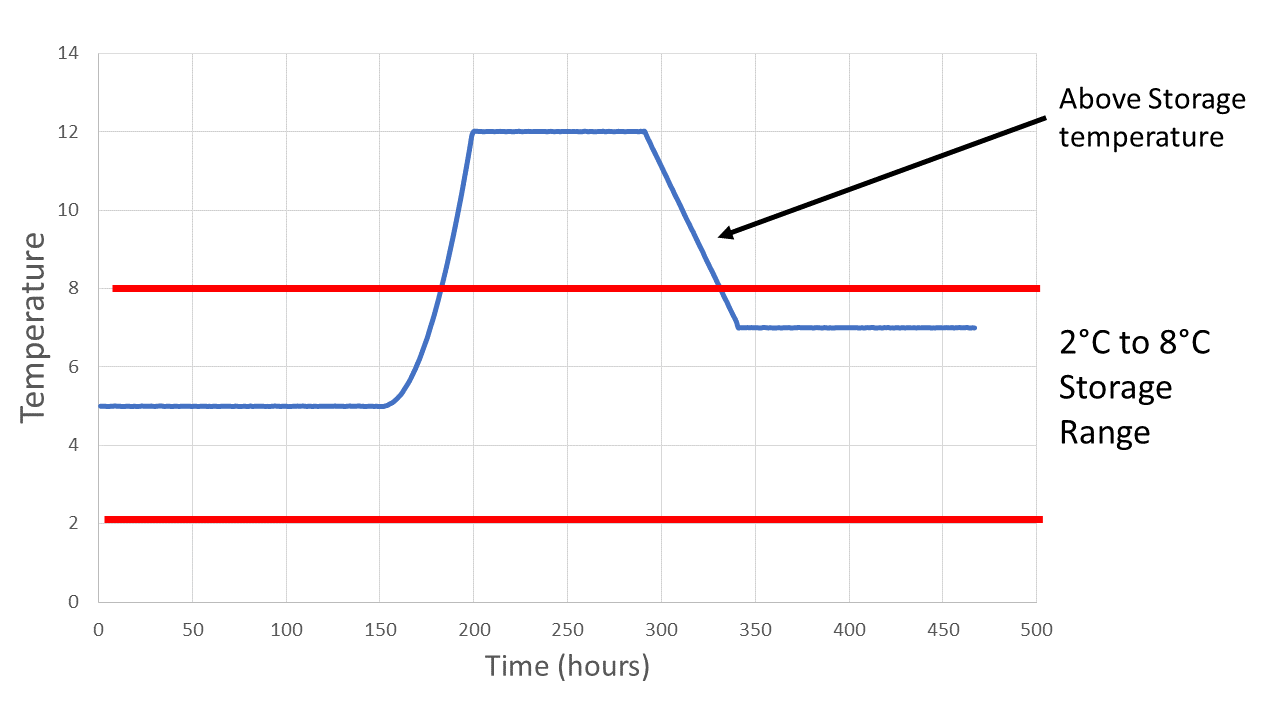 Excursion data depicts a temperature excursion event as a variance from the allowable storage temperature range.
Excursion data depicts a temperature excursion event as a variance from the allowable storage temperature range.As this experience demonstrated, the combination of robust data analysis tools and an efficient process makes it possible to deliver actionable information about temperature excursions to the right subject matter experts for effective analysis and quick decision-making. With the proper approach and tools in place, this biopharmaceutical company can respond to temperature excursions faster, reduce discarded shipments, and avoid repeat emergency shipments, saving over $1 million annually. The company also has greater confidence that the drug products it ships to patients will remain stable and safe throughout the cold chain.
1 https://www.shipabco.com/the-rules-for-shipping-pharmaceuticals-you-need-to-know/#:~:text=According%20to%20information%20from%20Shipwaves,temperature%20regulated%20shipping%20is%20important.
Are you aware of the open data files published online by the FDA? This...
read Details
Biopharma supply chain excellence starts by integrating your cold chain. By integrating your cold...
read Details
Introduction The integrity of cold chain management is pivotal in the demanding realm of...
read Details