Part 2: Expanding Shipping Temperatures Without Sacrificing Quality: A Guide to Transport Validation and PQ
Demonstrating Product Quality All pharmaceutical manufacturers must ensure that the safety and efficacy of...
read Details

With five FDA programs speeding drugs through approval to commercialization, the pace of scale-up is accelerating, shortening time to market by months or even years. To make the most of these expedited programs, drug developers should evaluate their entire process to eliminate bottlenecks and non-value-added tasks. This evaluation should include logistics, where planning temperature-control strategies as early as possible in the development process helps avoid delays and get medications to patients more quickly.
To put the trend toward speedy approvals in context, 200 of the 367 novel drugs approved by the FDA’s Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) between 2011 and 2018 used one or more of its programs. Of those 200, 12 percent received the Accelerated Approval designation. The remainder were expedited under Fast Track, Breakthrough Therapy, Regenerative Medicine Advanced Therapy, or Priority Review designations. In 2020 68% of novel drugs approved were designated one or more expedited category.
A growing percentage of those drugs require cold-chain or controlled room temperature shipping and handling. Specifically, in 2018, 44 percent of the FDA-approved drugs required controlled temperature handling, and the percentage increases each year. The 2019 Biopharma Cold Chain Sourcebook estimates that by 2023, approvals for temperature-controlled pharmaceuticals will increase 59 percent from 2017 figures.
Biologics are the main reason for this increase. Today’s novel therapeutics often are developed by innovative small to mid-sized biopharmaceutical companies that thrive on rapid development. To deliver therapeutics as quickly as possible, they often include rare and orphan diseases applications in their portfolios in the hopes of qualifying for expedited approval designations.
That’s an effective strategy, but it doesn’t follow the strategic template used for blockbuster drugs.
Cell and gene therapy advancement through expedited approval is a lot more challenging, especially when it comes to supply chain and cold chain.
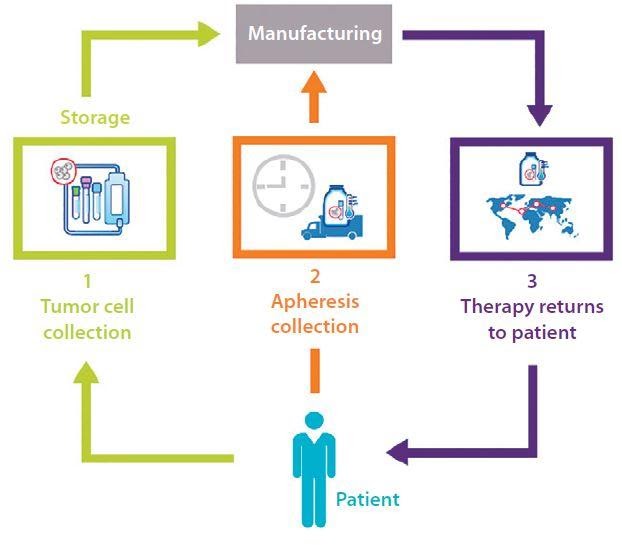
Cell and gene therapy both use the body’s own cells and genetic information to target and fight disease. Patients’ own (autologous) cells or the healthy donor’s (allogenic) cells or genetic material are extracted, then altered to create a personalized therapy and re-injected into the patient. The supply chain is a complex and unique closed loop, patient to patient, time sensitive and requires a just-in-time delivery process.
Biological material collected for autologous and allogeneic therapies must be shipped under stringent temperature controls, most likely cryogenic temperature. Shipment is under extremely tight timelines, and a critical chain of identity and custody must be maintained from patient to manufacturing and back to the patient.
To maximize the potential of today’s small-batch, personalized medicines, biopharmaceutical companies need managers who understand the newer, faster pace and start planning early. By planning early, they can deliver potentially-life-saving therapeutics to patients as quickly and effectively as possible.
Participating in one of the FDA’s expedited programs is only one step to commercializing and delivering innovative therapeutics quickly. There are additional steps drug developers can take to further speed the process. Consider these points:
Begin discussing your controlled temperature challenges during the drug development stage. Identify drug stability issues that may be affected by temperature, light exposure, shock, or vibration. Then research packaging solutions and data monitoring systems that meet the requirements of the data required by the regulators from your planned launch regions and the World Health Organization’s (WHO’s) Good Distribution Practices (GDP) and your own logistics team. By beginning early, you can find cost-effective solutions for all transportation options and speed time to market.
Use development time to create more stable formulations with formulation exposure to environmental hazards with simulated transportation. This simplifies packaging and logistics needs, and can reduce logistics expenses. Confirming formulation robustness can also can expand the market for novel and existing therapeutics (especially in the developing world, where refrigeration is unreliable).
At the same time, use the development time to identify potential cost savings throughout the cold supply chain. This may mean more efficient routes or logistics partners, or new or improved technologies that support delivery methods that were unthinkable until recently. For example, UPS and CVS deployed drones for the first time in November for last mile delivery, and Merck is testing drones for disaster response. Earlier, improved reefers and condition monitors have made maritime and rail shipping as reliable and economical for pharmaceuticals as they have been for more durable goods.
Develop and test an integrated closed loop cell therapy supply network starting with treatment centers where initial cell/DNA collections start, to manufacturers, couriers, distribution centers, and clinical trial sites and back to the treatment center and patient. Create a process map for scheduling collections and coordinating logistics between providers, and all sites involved in the supply chain.
Develop your products with good manufacturing processes (GMP) and continuous manufacturing (CM) in mind from the beginning. Designing processes for scale up from their inception minimizes the need to rework formulations and resubmit regulatory data when ingredients change. Likewise, CM enhances flexibility in batch-saving ways thanks to continuous optimization. Both strategies decrease development time from preclinical to commercial stages.
Implement, test and standardize closed-system processing and cryopreservation at collection sites to enable industrialization of advanced therapies through starting material standardization for manufacture and ultimately able to introduce GMP.
Developing a global regulatory cold chain strategy rather than a country-by-country approach is another time-saver. Once the markets are identified, determine their regulatory commonalities and differences. Then design the studies to meet the most rigorous regulatory requirements of those countries. (Note that even regions with similar requirements, like the U.S. and EU, are not perfectly aligned.) Developing a multi-nation strategy during the drug development process is faster and more cost effective than waiting until after commercialization in your primary markets.
Accelerated drug approval pathways are making significant impacts on patient health as well as the health of the companies that develop them. Those gains can be unwittingly sabotaged, however, when drug developers neglect the big picture.
Planning early for GMP, scalable and continuous manufacturing, global regulatory requirements, and temperature-controlled logistics, helps companies advance with certainty. Planning early helps minimize delays and setbacks and makes the most of the opportunities afforded by the FDA’s expedited drug programs.
To learn how Modality Solutions can help you with the big picture, contact us.
Demonstrating Product Quality All pharmaceutical manufacturers must ensure that the safety and efficacy of...
read Details
Introduction The integrity of supply chain management is pivotal in the demanding realm of...
read Details
Entering the U.S. market comes with a unique set of regulatory challenges, particularly when...
read Details