Lane Bracketing: How to Implement ISTA’s PCG-03 Recommendations
On July 22nd, 2022, ISTA released PCG-03: Performance Qualification (PQ) and Performance Verification (PV)...
read Details

On July 22nd, 2022, ISTA released PCG-03: Performance Qualification (PQ) and Performance Verification (PV) Best Practice Guideline. PCG-03 takes the first steps toward standardizing best practices for PQ strategy and execution for the biopharmaceutical industry, as it is the first document to compile strategies from thought leaders in the industry and provide a path forward for transport validation work.
We applaud ISTA for this useful and comprehensive list of considerations for determining your PQ strategy. However, there remains a clear gap on how to manage and implement their recommendations. The items listed within the document require further context to enable the industry to form a cohesive approach to PQ strategy.
This article series will seek to dive deep into the considerations listed by ISTA and discuss the implications and risks of each topic. Each article will contain a recommended tactical approach to each of these considerations:
As each article is released, we will link it in the Table of Contents above so you can navigate to each topic. In this article, the experts at Modality Solutions are diving into the Acceptance Criteria recommendations found in PCG-03.
The final, and most critical piece in designing your PQ studies is the determination of acceptance criteria. Inadequate acceptance criteria can jeopardize your entire study or lead to failing what should be fully compliant data.
Acceptance criteria are different than validation parameters (sometimes called qualification parameters). In short, validation parameters determine the validity of your study where acceptance criteria are how you judge the outcome of your study.
Validation parameters are the conditions that must be met to determine that the collected data is valid. Not complying with your validation parameters means that you cannot draw conclusions from the collected data. Typical validation parameters include all monitors having up to date calibrations, operators being trained on SOPs and the protocol, and completion of all study documentation.
I will cover four common acceptance criteria in this installment: temperature requirements, shipment duration, product quality, and loading/unloading.
The baseline acceptance criteria for PQs is that temperature is maintained at specification during the shipment. An example of this may be written as:
“Product temperatures must remain within 2 – 8 °C for the duration of the shipment.”
While this may seem straightforward, there are multiple considerations that must be built into your determination and evaluation of acceptance criteria.
The allowable temperature range for a PQ study should be determined based on product stability and label requirements. A common label requirement for shipping a monoclonal antibody finished product is 2 – 8 °C, for example. In a shipping PQ for this product, you might state in the acceptance criteria that product temperatures must be maintained at 2 – 8 °C for the duration of the shipment.
It is extremely important that the significant figures of the label requirement are understood. Virtually all label requirements are given in whole numbers whereas most temperature monitors record to at least the tenths place. Therefore, the range of 2 – 8 °C is not equivalent to 2.0 – 8.0 °C but 1.5 – 8.4 °C. The specification as a whole number allows for almost a full degree of flexibility to be added in. Recorded temperatures within 1.5 – 8.4 °C are fully compliant with the label requirement of 2 – 8 °C.
Therefore, acceptance criteria can be written as:
“Product temperatures must remain within 2 – 8 °C (1.5 – 8.4 °C) for the duration of the shipment.”
There are also certain events in the shipping process during which it is allowable for temperatures to fall outside of your specified range. The first of these events is shipper equilibration.
The shipper equilibration period is the time in which the recorded temperatures within a packed shipper are stabilizing. This equilibration period can be influenced by factors including temperature monitor conditioning, ambient air temperatures, monitoring location(s), and duration of the packout process. For cold shipments, this will often be seen as an initial cooling period before temperatures stabilize within your range, such as in the graph below.
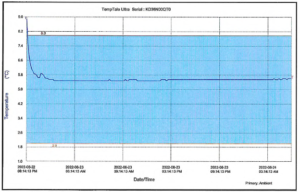
This initial equilibration period should be expected in most shipments and is not necessarily reflective of actual product temperatures. Therefore, it is accepted and recommended to include a set equilibration period that does not apply to acceptance criteria. Standard allowances are 60 minutes for small parcel shipments and up to 2 hours for larger pallet or truck shipments.
Acceptance criteria accounting for equilibration period can be written as:
“Product temperatures must remain within 2 – 8 °C (1.5 – 8.4 °C) for the duration of the shipment excluding an initial 60-minute equilibration period.”
There are three other common events during which temperature data should not be applied to acceptance criteria: loading, unloading, and inspection events.
Many facilities are not able to load and unload product within the specified temperature range. Recorded temperatures will therefore vary during loading and unloading as the monitors are exposed to ambient air. These operations can also be studied and are discussed in detail later in this post.
You should also include flexibility to allow for shipment inspection events, especially for international shipments. Even with all the proper paperwork and other controls in place, shipments that clear customs will be opened and inspected from time to time. This will again allow air at ambient temperature to enter the unit and influence the recorded temperatures.
Therefore, final acceptance criteria can be written as:
“Product temperatures must remain within 2 – 8 °C (1.5 – 8.4 °C) for the duration of the shipment excluding an initial 60-minute equilibration period and during loading, unloading, and inspection events.”

All of the example acceptance criteria written in the previous section used the wording “for the duration of the shipment.” However, that criteria did not include any requirements for the actual duration itself. So, how do you manage the duration of shipments used in a PQ study?
Oftentimes, stakeholders will lobby to include a requirement that tested durations match the qualified duration from a shipper’s Operational Qualification (for example, a 72-hour shipper is tested for 72 hours in a PQ). I do not recommend this strategy as this presents issues both in PQ scope and in the execution.
Tying back to PQ scope and purpose, extremes and worst-case functionality are tested in the OQ and do not need to be repeated in the PQ. The full range of performance cannot be verified in a PQ as you do not have control over the testing conditions: you cannot dictate ambient conditions nor ensure that the carrier will take the full allotment of time to deliver the package.
PQs only need to be representative shipments, not the worst-case lanes. The shipments used in the PQ study simply need to not be trivial compared to the typical shipment. For example, a shipment from a distribution center to a buyer two miles away should not be used to cover all domestic shipments.
Therefore, if shipment duration is to be included in the acceptance criteria, it should focus on ensuring that the study shipments are reasonably representative. An example of this acceptance criteria for a shipping lane that may take up to two days would be:
“Three shipments must be included for this study that have a total duration of at least 24 hours”
As mentioned in the previous section, product may be exposed to temperatures outside of specification during the loading and unloading processes. While the temperatures recorded during these processes should not be tied to acceptance criteria, you may need to look at the total duration of time out of temperature.
Procedural controls may dictate that start and end times of operations outside of temperature specification be recorded. If true, you should always know exactly how long the product was exposed to these conditions. In that case, the duration of the loading and unloading operations is not required as acceptance criteria in your PQs because it is always recorded. You may still choose to include it to ensure that piece of the process is operating as intended.
If, however, these durations are not recorded, it is recommended to include the documentation of the loading and unloading operations in your PQ. The allowable duration for these operations will depend on factors including product stability and expected ambient conditions. A common standardized allowance for loading and unloading operations is sixty minutes each.
The last consideration for acceptance criteria I will discuss in this installment is product quality. There is an expectation in commercial filings that you demonstrate your product quality is not affected by shipping and will arrive to patients with safety and efficacy intact. Historically, drug developers met this expectation by including analytical testing of product shipper in their PQ studies.
This historical approach has many flaws and is now being scrutinized by regulators and industry groups. Primarily, real world shipping in a PQ cannot stress the product at the process extremes. Similar to the differences between shipping OQs and PQs, you cannot control the input of hazards on the product during real shipping to adequately determine worst-case performance. The only way to ensure that your product is robust to worst-case shipping hazards is through the use of transport simulation studies which you can read about here.
There are also flaws in this approach from a supply chain viewpoint as well. Attempting to tie product quality metrics to your shipping PQs creates cascading complications in how the studies must be designed, implemented, and managed post-execution. For example, you may now need to design the chosen shipments to be the absolute longest shipments in your supply chain, or use mock shipments to destinations further than your current network to allow for future flexibility.
If product quality is tied into a shipping PQ, then that means product quality is also tied to a thermal shipping system. Including product quality in your PQs effectively locks you into the exact shipping systems, lanes, and conditions used for testing lest you need to repeat a full suite of analytical testing. In essence, this erases all flexibility and resiliency from your supply chain at a time when supply chain uncertainty is rampant. Product quality demonstration should be kept completely separate from thermal control in your validation programs.
With the implementation of acceptance criteria in your study, the PQ design process is complete! Hopefully, this article series has you feeling well equipped to design and execute PQs across your supply chain. But what do you do once the PQs have been completed? The sixth and final installment in this series will answer those as we focus on Management of Validated Lanes.
This installment focused on the monitors used to collect the data. Gathering the correct data, however, is only the first step. The next step is how to analyze the data collected during a PQ to make a final determination on the state of the shipping process. The subsequent installment will focus on acceptance criteria for PQs, including non-temperature data. Catch up on the first four articles in the series below:
Modality Solutions is a leading provider of innovative and comprehensive solutions for the biopharmaceutical industry. Our team of experts has extensive experience in the industry and works closely with national and international regulators to ensure that our clients stay ahead of the latest industry developments and best practices. We pride ourselves in providing tailored solutions to meet the unique needs of our clients, and we are committed to helping organizations achieve their PQ goals. Contact us for more information on how we can help your organization navigate the complex world of biopharmaceutical transportation. And don’t forget to follow us on LinkedIn to stay updated on our latest developments.
On July 22nd, 2022, ISTA released PCG-03: Performance Qualification (PQ) and Performance Verification (PV)...
read Details
On July 22nd, 2022, ISTA released PCG-03: Performance Qualification (PQ) and Performance Verification (PV)...
read Details
On July 22nd, 2022, ISTA released PCG-03: Performance Qualification (PQ) and Performance Verification (PV)...
read Details