Scope & Timing: How to Implement ISTA’s PCG-03 Recommendations
On July 22nd, 2022, ISTA released PCG-03: Performance Qualification (PQ) and Performance Verification (PV)...
read Details

On July 22nd, 2022, ISTA released PCG-03: Performance Qualification (PQ) and Performance Verification (PV) Best Practice Guideline. PCG-03 takes the first steps toward standardizing best practices for PQ strategy and execution for the biopharmaceutical industry, as it is the first document to compile strategies from thought leaders in the industry and provide a path forward for transport validation work.
We applaud ISTA for this useful and comprehensive list of considerations for determining your PQ strategy. However, there remains a clear gap on how to manage and implement their recommendations. The items listed within the document require further context to enable the industry to form a cohesive approach to PQ strategy.
This article series will seek to dive deep into the considerations listed by ISTA and discuss the implications and risks of each topic. Each article will contain a recommended tactical approach to each of these considerations:
As each article is released, we will link it in the Table of Contents above so you can navigate to each topic. In this article, the experts at Modality Solutions are diving into the Lane Bracketing recommendations found in PCG-03.
After reading the previous post, you should understand the scope of your PQ studies and when you should execute them. In this post, we will detail how to apply your strategy to the various shipping lanes for your biopharmaceutical product, including how to bracket the studies for multiple shipping lanes.
PQs have historically been completed with at least three shipments per unique lane: more extensive or more complex shipping networks can quickly get bogged down by a massive number of shipments required under protocol. Bracketing allows for a single PQ study to apply to multiple shipping lanes. Effectively applying a bracketing strategy can significantly reduce the complexity and effort required to execute your PQ studies.
Before beginning, your supply chain should be well understood: a process map is a helpful tool to accomplish this. With the lanes all set out before you, we can begin unraveling the shipping processes and understand where bracketing does and does not apply.
The three critical considerations for choosing a bracketing strategy for multiple lanes are:
Let’s explore this in a situation where a single origin site is shipping to multiple destinations. The figure below shows an example shipping network from a Packaging Facility to distributors or 3PLs located in the US, the UK, and the EU.
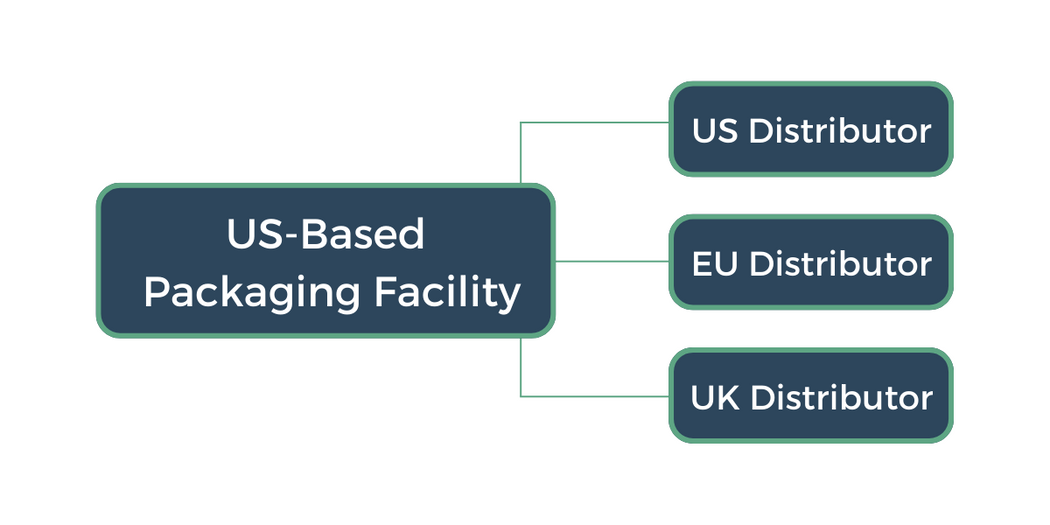
The most conservative approach here would be to complete a unique PQ study for each lane: at three shipments per study, this is nine shipments to complete under protocol. This design could be even larger if different thermal shipper options are used in any single lane (we will cover this in Part III of this blog series, stay tuned).
These lanes may be bracketed, meaning that a single study could cover two or even all three lanes. Correctly applying a bracketing strategy can save time and money while still adequately demonstrating that the shipping process operates in a state of control.
For this hypothetical situation, let’s assume the following:
Therefore, this study can be executed with three standard shipments to any distributors in Europe or the UK. The only caveat comes from the third consideration around special operations.
If the operations during transit to arrive at a specific facility are significantly more complex, there may need to be additional scrutiny paid to that lane. For example, if the UK facilities can receive and store product while it is awaiting customs clearance, but the European sites cannot, then at least one shipment should occur to the European sites to ensure that the added complexity is included in the PQ execution.
The second way lanes can be bracketed is when multiple sites execute a comparable shipping process. For example:
As before, the key factor in applying this bracketing strategy is the comparability of the shipping process. This strategy will be a risk-based decision and should encompass more than just the process of getting a package out the door. There are supporting processes that factor into the success of your product shipments: training, change management, work aids, etc. These all must be considered for the potentially bracketed sites to determine comparability.
We will explore this bracketing strategy through a typical apheresis supply chain for cell therapies. The apheresis is collected at a network of clinical sites and shipped to a central manufacturing facility. For this scenario, we will assume that all collection sites are using the same thermal shipper and that their shipping processes can be considered comparable. Potential leverage for justifying the comparability of the processes can include the same work instructions used at each site, accreditation(s), and audits.
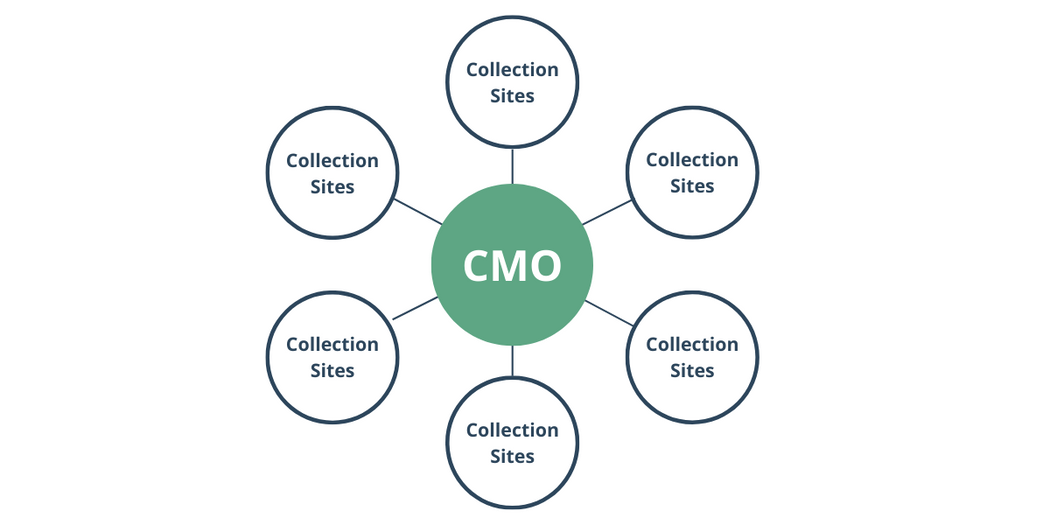
Rather than completing three shipments from each facility, one of the following strategies should be employed:
This methodology shows the repeatability of the shipping process from a single location while including the shipments from other sites to demonstrate comparability.
Sending one shipment from each facility demonstrates the comparability of the various shipping processes. A total of at least three shipments should be completed when using this strategy to adequately demonstrate the repeatability of the process.
This methodology is best used for complex supply chains with a multitude of origin sites. Process comparability is justified but not demonstrated through testing at each location. By stating that the shipping process at all sites is effectively the same, this PQ can operate with a traditional three shipment approach from any of the sites in the supply chain.
Using any of these methodologies confirms the goal of the PQ: demonstrating that the shipping process operates in a state of control. Comparability is either demonstrated or justified in the testing protocol and report. The bracketing strategy ensures that the multiple parties executing the comparable shipping process have data showing their process performance.
The examples within this article can be applied to greatly reduce the total amount of shipments needed for PQ execution. Bracketing various shipping lanes is an effective methodology for demonstrating that the shipping process operates in a state of control. However, the examples given here made one crucial assumption: that the same thermal shipper is used. Our next article will cover how to adjust your PQ strategy to account for multiple types of thermal shippers being used for shipping.
Modality Solutions is a leading provider of innovative and comprehensive solutions for the biopharmaceutical industry. Our team of experts has extensive experience in the industry and works closely with national and international regulators to ensure that our clients stay ahead of the latest industry developments and best practices. We pride ourselves in providing tailored solutions to meet the unique needs of our clients, and we are committed to helping organizations achieve their PQ goals. Contact us for more information on how we can help your organization navigate the complex world of biopharmaceutical transportation. And don’t forget to follow us on LinkedIn to stay updated on our latest developments.
On July 22nd, 2022, ISTA released PCG-03: Performance Qualification (PQ) and Performance Verification (PV)...
read Details
On July 22nd, 2022, ISTA released PCG-03: Performance Qualification (PQ) and Performance Verification (PV)...
read Details
On July 22nd, 2022, ISTA released PCG-03: Performance Qualification (PQ) and Performance Verification (PV)...
read Details