Unlocking FDA Insights: Exploring Open Data Files for Shipping Validation
Are you aware of the open data files published online by the FDA? This...
read Details

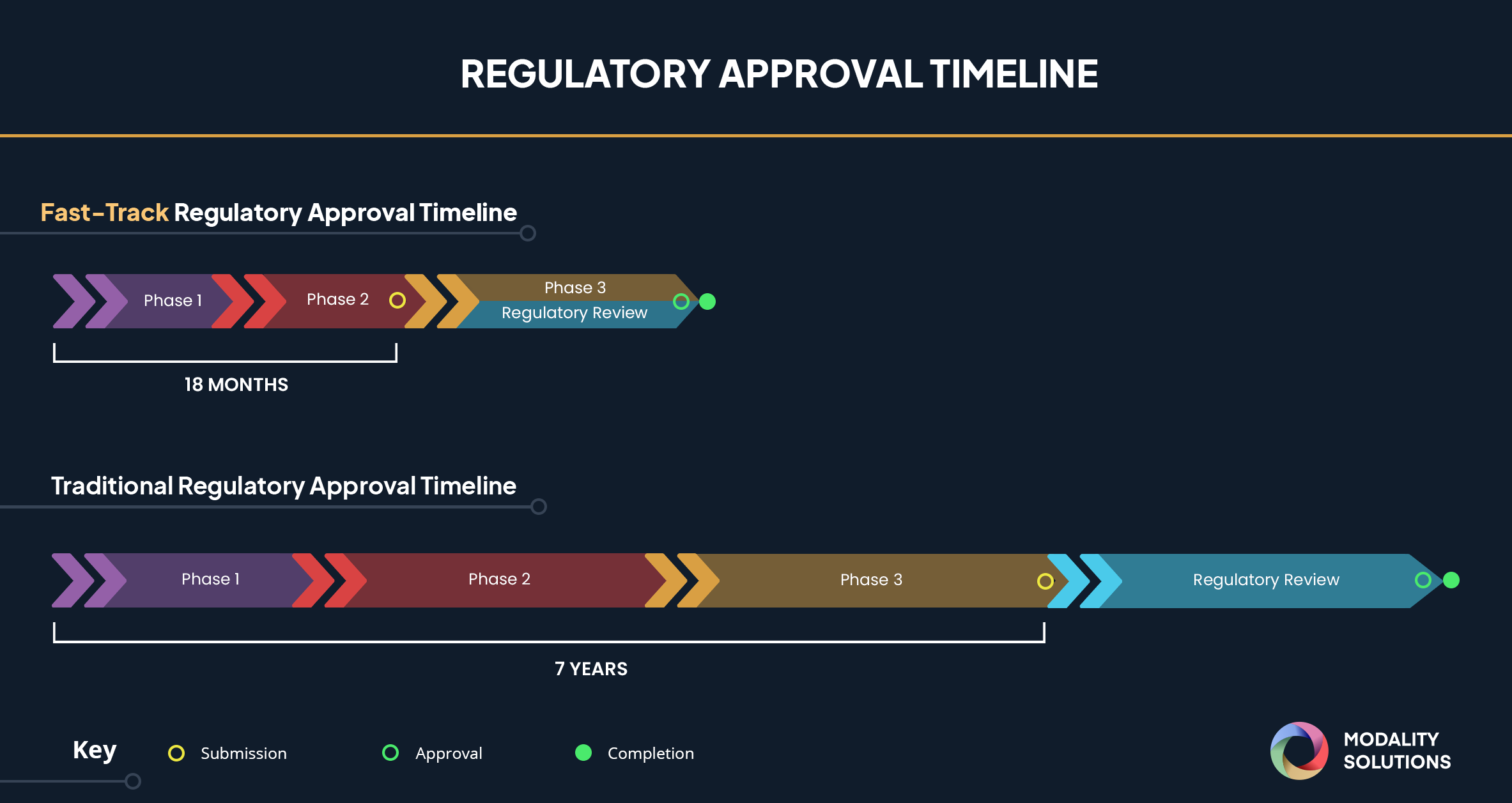
The Coronavirus Treatment Acceleration Program launched by the FDA in response to COVID-19, is a Supercharged Fast-Track Approval process that reduces the Fast-Track Approval timeline from 18 months to as soon as possible in order to get treatment to the patients as fast as possible.
Can the pharmaceutical supply chain adapt to the hyperspeed of CTAP approvals?
A vaccine traditionally takes 7-8 years to develop from the laboratory tables to the patient. The COVID-19 pandemic is inducing a rush of clinical trials for existing drugs and biologics to see if they can be potentially used against the virus, as well as initiating novel approaches, including mRNA, DNA vaccine, and vectors to be tested through clinical trials.
“The list of biopharmaceutical companies rapidly developing vaccines is growing in response to the novel Coronavirus (COVID-19) pandemic. Moderna Therapeutics has a messenger RNA (mRNA) in Phase I trials, Inovio Pharmaceuticals’ DNA vaccine entered human trials the first week of April, while others including Novovax, which is developing a vaccine based on its recombinant protein nanoparticle platform.” (1)
Innovators are doing their part, and so is the FDA. Breakthrough Therapy Designation or Fast-Track Approval was implemented by the FDA for accelerating treatment development for serious conditions such as the Coronavirus pandemic. This pandemic prompted the FDA to further supercharge the process by creating the Coronavirus Treatment Acceleration Program (CTAP). The FDA intends to use all of the regulatory flexibility granted to it by Congress to ensure the most efficient and timely development of vaccines to fight COVID-19.
The vaccines or treatments aimed at treating high patient populations globally to control a pandemic will require millions if not billions of doses. Most of the leading COVID-19 vaccine candidates are using novel approaches, including mRNA, DNA vaccine, and vectors that are highly sensitive to environmental hazards occurring during transportation such as temperature, shock, and vibration.
Is your current supply chain ready for the volume, global distribution, and speed of new therapy approvals? Early implementors will rise to the challenge.
Modality Solutions has successfully worked with clinical trial supply chain issues for the Ebola vaccine, and we have the testing capabilities to evaluate the effects of environmental hazards throughout the supply chain. We also have the expertise of integrating existing or creating new supply chain protocols for therapies developed to treat the Coronavirus.
References
Novel R&D approaches and the need for flexibility make single-use key in tackling COVID-19
https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap
Are you aware of the open data files published online by the FDA? This...
read Details
Biopharma supply chain excellence starts by integrating your cold chain. By integrating your cold...
read Details
Introduction The integrity of cold chain management is pivotal in the demanding realm of...
read Details